Abstract
Introduction: Polycythemia vera (PV) is a myeloproliferative neoplasm (MPN) clonally arising from a malignant hematopoietic stem cell (HSC) bearing a driver mutation, most frequently JAK2V617F. JAK2V617F in PV has only rarely been observed in lymphocytes, which is surprising since HSCs give rise to all hematopoietic lineages. We propose two alternative hypotheses to explain this observation, either (A) lymphopoiesis in adults with PV occurs prior to the acquisition of JAK2V617F in HSCs, followed by relatively slow attrition of long-lived unmutated mature lymphocytes, or (B) JAK2V617F interferes with lymphopoiesis from HSCs. The direct and indirect effects of MPNs on lymphopoiesis is a topic of study relevant to the inflammation-mediated complications that characterize these diseases.
Objective: To critically test the competing hypotheses explaining the lack of JAK2V617F entry into T lymphoid populations in a large cohort of patients with PV.
Methods: Peripheral blood was collected from 50 patients with JAK2V617F PV according to IRB-approved procedures and fractionated into myeloid and lymphoid subpopulations via fluorescence-activated cell sorting (FACS). To track the malignant clone across lineages, JAK2V617F mutation allele frequency (MAF) was measured in each subpopulation by droplet digital PCR (ddPCR). Hypothesis (A) predicts that PV duration and patient age at diagnosis correlates with JAK2V617F MAF in lymphocytes, so this was tested in our cohort by linear regression. Hypothesis (B) was tested by determining the extent to which T cell differentiation is impaired in JAK2V617F mutated, primary CD34+ hematopoietic stem and progenitor cell (HSPC) samples isolated from 15 patients with PV. HSPCs were differentiated in vitro into T cell progenitors using a Notch signaling-based culture system. As a control, HSPC differentiation toward the erythroid lineage was performed in parallel for each sample. The effect of JAK2V617F on differentiation was tracked by MAF using ddPCR in progenitor subpopulations obtained by FACS along validated T cell and erythroid ontogenies (adapted from Awong et al. Blood 2009 and Fajtova et al. Leuk Lymphoma 2013, respectively).
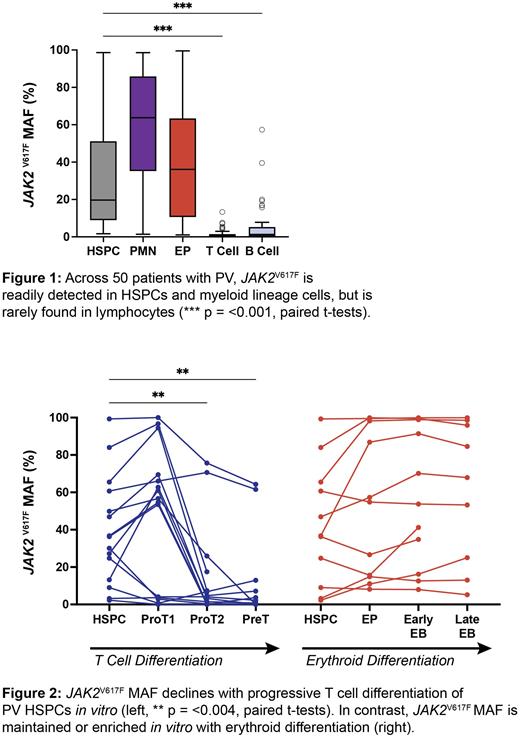
Results:JAK2V617F was readily detected in HSPCs, granulocytes (PMNs) and erythroid progenitors (EPs) across our PV cohort but was detected only at very low levels in T cells (median MAF 0.55%) and B cells (median MAF 1.3%) (Figure 1). JAK2V617F MAF in HSPCs was positively correlated with patient age (+0.77% MAF per year of age, p = 0.007) and duration of PV (+1.7% MAF per year of PV, p = <0.001) but MAF in T cells was unrelated to either (-0.003% MAF per year of age and +0.03% MAF per year of PV, p = 0.92 and 0.55, respectively). The cohort included patients 30-90 years of age with PV duration ranging from 0-30 years. Given these time frames that likely exceed the lifespans of the longest-lived lymphocytes, with recent estimates that JAK2V617F mutations are acquired in HSCs decades prior to clinical MPN diagnoses (Williams et al. Nature 2022), it is unlikely that all T lymphopoiesis occurred prior to the acquisition of JAK2V617F across our cohort. Thus, Hypothesis (A) appears implausible.
We tracked JAK2V61F MAF within 3 immunophenotypically defined T cell progenitor subpopulations that developed during in vitro differentiation of PV HSPCs. All 15 PV samples could be differentiated toward T cells. However, we observed a marked decrease in JAK2V617F MAF as T cell differentiation progressed (Figure 2). In contrast, JAK2V617F MAF was maintained or increased during in vitro erythroid differentiation toward erythroblasts (EB). These results indicate that JAK2V617F exerts a selective bias against T cell commitment in PV, thereby supporting Hypothesis (B).
Conclusion:JAK2V617F mutated T cells are seldom observed in patients with PV and JAK2V617F HSPCs exhibit impaired T cell differentiation in vitro. Patients with MPN appear to have deficient immune surveillance that manifests with increased risks of infection (Landtblom et al. Leukemia 2021) and second malignancies (Frederiksen et al. Blood 2011), independent of therapy. Our study suggests that impaired T cell differentiation could contribute to such risks. Further study is under way to define actionable mechanisms through which JAK2V617F interferes with T cell differentiation. Additional correlative studies will report the prognostic implications of lymphocyte JAK2V617F MAF in PV.
Disclosures
Scandura:MPN-RF: Research Funding; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; CR&T: Research Funding; European Leukemia net: Honoraria, Other: Travel fees; Sumitomo Pharma Oncology, Inc: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal